RESEARCH
The Seo research group is interested in discovering the fundamental mechanisms regulating essential metals in human health and disease.
Iron and manganese are essential nutrients for our life. Disturbances of iron and manganese metabolism, caused by genetics, dietary, or environmental factors, can have detrimental impacts on human health. Iron deficiency, for example, is the most prevalent nutrient deficiencies in the world, affecting an estimated 2 billion individuals, mainly young children and women. In contrast, having too much iron (i.e., iron overload) is increasingly being recognized a public health concern. Hemochromatosis is a frequent genetic disorder characterized by the accumulation of excess iron across tissues. Untreated hemochromatosis can be fatal due to organ failures. As many as 1 in 200 Americans are at genetic risk for developing hemochromatosis. While manganese deficiency can result in distinct neurodevelopmental conditions, manganese toxicity can lead to brain manganese accumulation and a parkinsonian-like disorder.
Despite the prevalence and adverse health effects associated with iron and manganese related disorders, our understanding of how iron and manganese are regulated at the molecular level is limited. The Seo lab focuses on identifying and characterizing the roles of iron and manganese in related disorders at the molecular, cellular, and physiologic levels. This research will ultimately help to identify therapeutic targets for treating disorders related to iron and manganese metabolism.
Current Projects

Manganese in Physiology
The goal of this project is to define the physiological roles of Mn. Currently, we focus on the roles of Mn in the intestines and its contribution to the development of inflammatory bowel disease (IBD). IBD, including Crohn’s disease and ulcerative colitis, is a group of chronic inflammatory disorders of the gastrointestinal tract. The incidence of IBD is rapidly increasing worldwide, affecting over 3 million Americans. At present, no cures exist, and treatment options are limited, leading to a pressing need to develop new therapeutic targets for IBD. We are currently working on the mechanistic roles of Mn in the intestine and IBD.
Manganese in Brain
The goal of this project is to understand how Mn regulation supports normal and pathological brain development and functions. Recent epidemiological surveys have found that both low and high Mn levels are associated with cognitive and behavioral impairment in children. Moreover, exposure to high levels of Mn can lead to brain Mn accumulation and a parkinsonian-like disorder known as manganism or Mn poisoning. Thus, the correlation between Mn dysregulation and brain malfunction in humans is well established. However, the causal relationship between the two remains to be determined. We are currently working on the mechanistic roles of Mn in the brain and neurological disorders.
Iron Imbalance Disorders
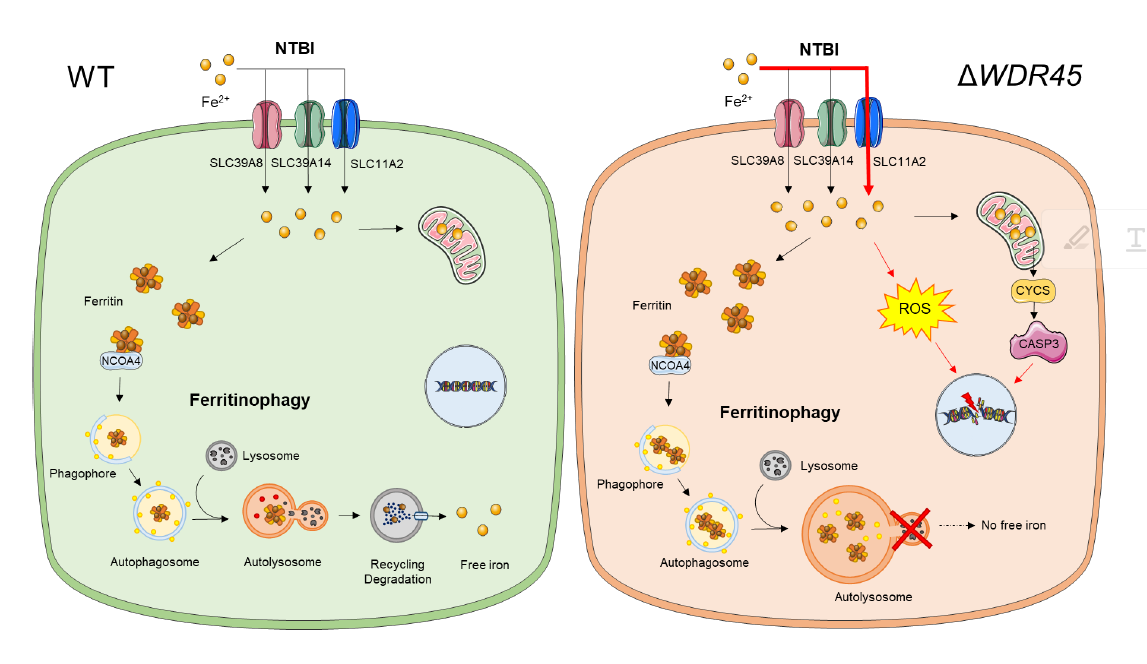
Iron imbalance underlies various diseases, ranging from anemia to neurodegeneration. The goal of this project is to examine the mechanistic underpinnings of iron imbalance disorders. Currently, we are interested in neurodegeneration with brain iron accumulation (NBIA). This disorder is a clinically and genetically heterogeneous group of neurodegenerative diseases characterized by an abnormal accumulation of brain iron and progressive degeneration of the nervous system.
β-propeller protein-associated neurodegeneration (BPAN) is a recently identified subtype of NBIA. BPAN patients display global developmental delays in infancy/early childhood, followed by neurological deterioration in early adulthood with progressive dystonia, parkinsonism, cognitive decline, and seizures. Excess iron, which can be visualized by an MRI of the brain, mainly accumulates in the discrete brain regions, such as the globus pallidus and the substantia nigra. The molecular and cellular mechanisms of brain iron overload in BPAN remain unclear, which makes it difficult to design rational therapeutics to treat these conditions. We are currently working on the molecular and cellular mechanisms underlying NBIA disorders.
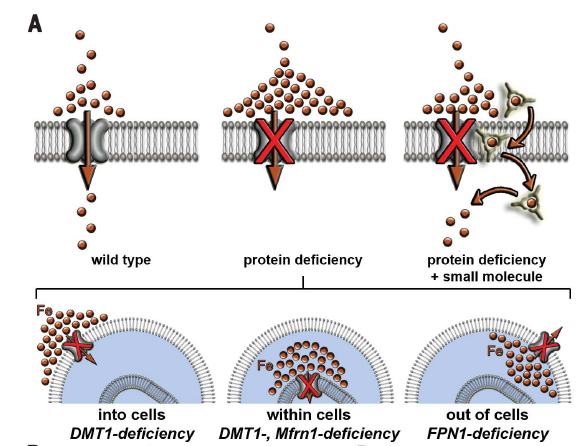
Iron Mobilization
The goal of this project is to build a foundation of the potential clinical use of a small molecule as a novel therapeutic approach for treating iron imbalance disorders. Deficiencies or dysfunction of proteins that promote transmembrane iron transport cause more than twenty-five human diseases affecting millions worldwide. For example, partial or complete loss-of-function mutations in FPN1 cause hemochromatosis type IV also known as Ferroportin disease, and acquired deficiencies of FPN1 cause Anemia of Inflammation in millions of patients with autoimmune disorders. Current therapies fail to address the underlying molecular defect – i.e. the missing capacity to transport iron across lipid membranes, and the most effective treatment is still regular phlebotomy or blood transfusions. We are currently working on a small molecule iron mobilizer for better understanding and possibly treating iron imbalance disorders.